Process Overview
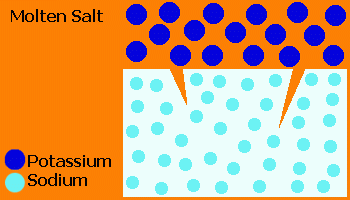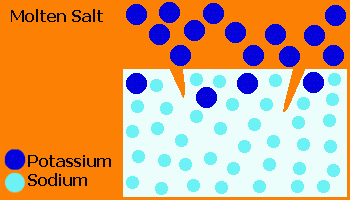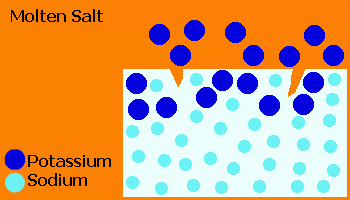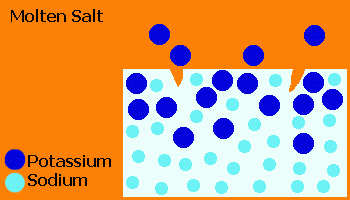
In the Ionex™ process, glass is treated in a molten salt (usually KNO3) causing ion exchange of small Na+ ions from the glass with larger K+ ions from the salt.
The "stuffing" effect of the large K+ ions forces the surface into a compression, producing a closure stress on cracks.
Since glass fails in tension only generally originating at a surface, an externally applied tensile stress must first overcome the surface compression before the occurrence of crack growth.
It is also more difficult for new cracks to form during handling because of increased abrasion resistance.
This means that glass is effectively stronger; the strength is increased in relation to the magnitude of compressive stress at the crack tip.
Since the treatment is carried out at temperatures just below the glass transition temperature, the product does not suffer any significant deformation or optical distortion.
In Saxon's Ion-Klad™ process, glass is subjected to longer process. This allows minimum strengths to be increased by creating a greater depth of surface compression (case depth), going beyond the deeper flaws.